Biochemistry is Your Friend. For Reals!
If you mention fermentation to the average person, it usually conjures up images of beer. However, in the science world fermentation is the process by which organisms get energy from the environment. These biochemical pathways existed long before we took advantage of their waste products: alcohol and lactic acid. In cheesemaking, we’re interested in the production of lactic acid to coagulate our milk to form curds.
Fermentation is the process that breaks down the nutrients taken in to the cell and converts them to ATP, which is the most common and valuable molecular form of energy used by all living organisms. The typical starting products for fermentation are sugars like glucose, sucrose, and in the case of cheese, lactose. However, cells can also make ATP from fatty acids and other organic molecules too.
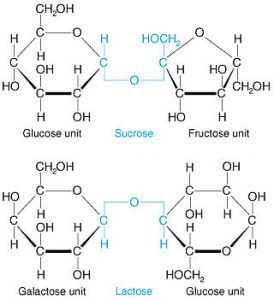
Glucose is the cash of the molecular energy world. It’s a six-carbon sugar that is used as the starting material for ATP generation, and it requires no further processing in order to get it going down the path of fermentation. Since it’s only composed of one molecule, it’s called a monosaccharide. Sucrose and lactose, on the other hand, need to be broken down first because they are disaccharides. That is, they are composed of two sugar subunits:
When sucrose is broken down, you get one molecule of glucose and one molecule of fructose. Lactose is broken down in to one molecule of galactose and one molecule of glucose. Since glucose is the preferred starting material in the generation of ATP, we’ll focus on that first.
In order to make ATP, glucose must first be cleaved in to two molecules of pyruvate in a process called glycolysis.


This process occurs in humans, too! However, human cells are also capable of a process called aerobic respiration, of which pyruvate is the starting material. It requires oxygen and produces a lot of ATP and other molecular energy currency such as NADH.
Our little cheesemaking bacteria are not so lucky. They rely entirely on the fermentation process for making ATP. The production of lactic acid (in the case of lactic acid fermentation) or alcohol (from alcohol fermentaion) from pyruvate is done entirely to make NAD+, which we need for the fermentation process.
So how is the same process able to deliver us delicious alcohol on one side and tasty cheese on the other? It’s all in the biochemistry.
Lactic Acid Fermentation
Lactic acid fermentation is the most basic type of fermentation. When we break glucose in to the two molecules of pyruvate to generate ATP, we transfer electrons to NAD+ in order to make NADH. However, there isn’t a lot of NAD+ hanging around in the cell. Luckily, with the help of the enzyme lactate dehydrogenase, pyruvate accepts electrons directly from NADH in order to generate the NAD+ that we need to break down more glucose. So you see, the balance of NADH and NAD+ can remain relatively constant in the cell while we crank out as much ATP as the incoming glucose allows. Rad!
In the simplest form of lactic acid fermentation, called homolactic fermentation, the two molecules of pyruvate are converted in to two molecules of lactate (lactic acid). Some organisms are also capable of heterolactic fermentation, which results in the production of one molecule of lactate, one molecule of ethanol, and one molecule of CO2.
Lactic acid fermentation occurs in bacteria and also in our cells as well. You might be familiar with the burning sensation you get when you work out really hard. That’s a result of your muscles not getting enough oxygen. So instead of using the aerobic respiration seen above that generates tons of ATP, your muscles pack it in and can only use glycolysis. The byproduct – lactic acid – is what causes the burn. If our muscles instead chose to undergo alcohol fermentation, we would get drunk exercising. Science!
In cheesemaking, the production of lactic acid by bacteria is one of the ways in which we get curds to form. This is the primary method of coagulation for chèvre and cream cheese, for instance. It’s also the reason that we say milk goes “sour” when we’ve kept it in the fridge for too long. Not that any of us has ever done that, of course.
Alcohol Fermentation
Some bacteria and yeasts can undergo alcohol fermentation. While production of lactic acid relies on only one enzyme, lactate dehydrogenase, alcohol fermentation requires two: pyruvate decarboxylase and alcohol dehydrogenase. Pyruvate decarboxylase converts pyruvate in to an intermediate, called acetaldehyde, which is then converted in to alcohol (ethanol) and carbon dioxide by alcohol dehydrogenase.
An overview of both processes, collectively known as anaerobic metabolism looks like this:
Interestingly enough, alcohol dehydrogenase is the same enzyme that your body uses to break down alcohol when you drink it.
Now if we put together the entire process, from glucose to lactic acid, in to one figure it looks something like this:
Biochemistry Truly is Your Friend
When all is said and done, the same processes that lead to the making of cheese and beer are the same as those that lead to making energy for you to run – or in my case, make cheese. I take that as a sign that we should be drinking lots of beer and eating lots of cheese. Do it for science!